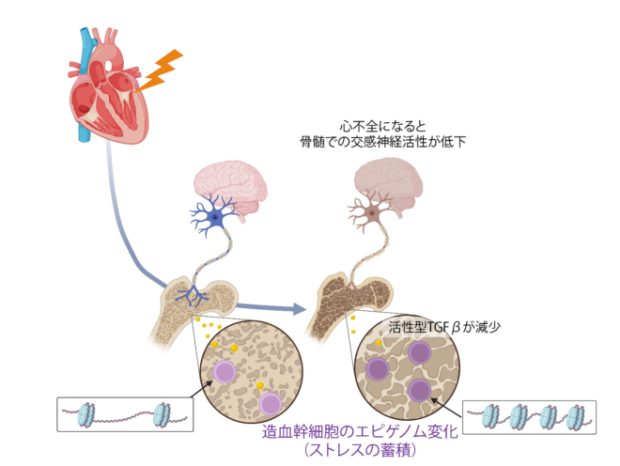
Nakayama Y, Fujiu K, Oshima T, Matsuda J, Sugita J, Matsubara TJ, Liu Y, Goto K, Kani K, Uchida R1, Takeda N, Morita H, Xiao Y, Hayashi M, Maru Y, Hasumi E, Kojima T, Ishiguro S, Kijima K, Yachie N, Yamazaki S, Yamamoto R, Kudo F, Nakanishi M, Iwama A, Fujiki R, Kaneda A, Ohara O, Nagai R, Manabe I, Komuro I, Science Immunology, 2024 May 24;9(95):eade3814. doi: 10.1126/sciimmunol.ade3814
Fujiu K and Manabe I, Nerve–macrophage interactions in cardiovascular disease. Int Immunol. 2021 doi:10.1093/intimm/dxab036
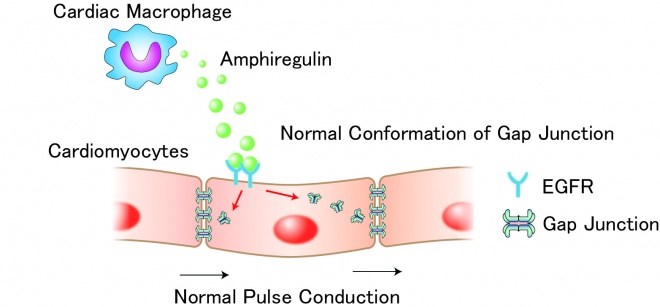
Sugita J, Fujiu K, Nakayama Y, Matsubara T, Matsuda J, Oshima T, Liu Y, Maru Y, Hasumi E, Kojima T, Seno H, Asano K, Ishijima A, Tomii N, Yamazaki M, Kudo F, Sakuma I, Nagai R, Manabe I, Komuro I. Cardiac macrophages prevent sudden death during heart stress. Nat Commun. 2021 Mar 26;12(1):1910. doi: 10.1038/s41467-021-22178-0.
Hasumi E, Fujiu K, Chen Y, Shimuzu Y, Oshima T, Matsunaga H, Matsuda J, Matsubara TJ, Fukuma N, Yuziang Liu, Sugita J, Nakayama Y, Saga A, Oguri G, Kojima T, Maru Y, Shoda M, Komuro I, Heart failure grading using single-lead electrocardiography, medRxiv. doi: 10.1101/2020.10.08.20209700
Nakayama Y, Fujiu K, Yuki R, Oishi Y, Morioka M, Isagawa T, Oshima T, Matsubara T, Sugita J, Kudo F, Kaneda A, Endo Y, Nakayama T, Nagai R, Komuro I, Manabe I, A long noncoding RNA regulates inflammation resolution by mouse macrophages through fatty acid oxidation activation, Proc. Natl. Acad. Sci. USA, Jun 2020, 202005924. doi: 10.1073/pnas.2005924117
Adachi H, Kawamura Y, Nakagawa K, Horisaki R, Sato I, Yamaguchi S, Fujiu K, Waki K, Noji H, Ota S, Use of Ghost Cytometry to Differentiate Cells with Similar Gross Morphologic Characteristics, Cytometry, Cytometry A. 2020 Apr;97(4):415-422. doi: 10.1002/cyto.a.23989.
Asakawa M, Itoh M, Suganami T, Sakai T, Kanai S, Shirakawa I, Yuan X, Hatayama T, Shimada S, Akiyama Y, Fujiu K, Inagaki Y, Manabe I, Yamaoka S, Yamada T, Tanaka S, Ogawa Y, Upregulation of cancer-associated gene expression in activated fibroblasts in a mouse model of non-alcoholic steatohepatitis, Sci Rep, 2019 Dec 20;9(1):19601. doi: 10.1038/s41598-019-56039-0.
Okamoto H, Yoshimatsu Y, Tomizawa T, Kunita A, Takayama R, Morikawa T, Komura D, Takahashi K, Oshima T, Sato M, Komai M, Podyma-Inoue KA, Uchida H, Hamada H, Fujiu K, Ishikawa S, Fukayama M, Fukuhara T, Watabe T. Interleukin-13 receptor α2 is a novel marker and potential therapeutic target for human melanoma. Sci Rep. 2019 Feb 4;9(1):1281.
Ota S, Horisaki R, Kawamura Y, Ugawa M, Sato I, Adachi H, Yamaguchi S, Fujiu K, Waki K, Noji H, Response to Comment on “Ghost cytometry”, Science, 2019, Vol. 364, Issue 6437, eaav3136.
Ota S, Horisaki R, Kawamura Y, Ugawa M, Sato I, Hashimoto K, et al. Ghost cytometry. Science. 2018 Jun 15;360(6394):1246-51.
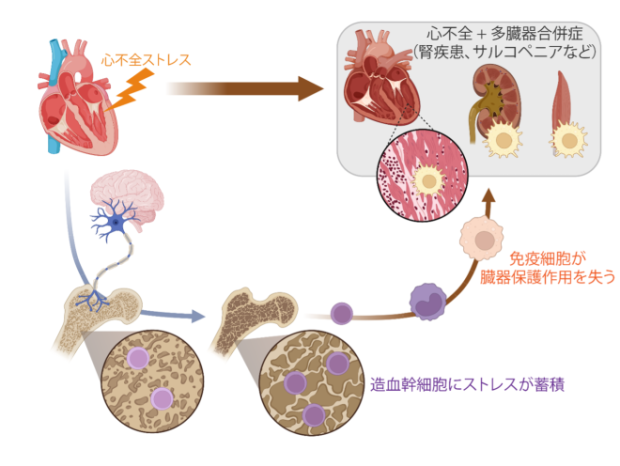
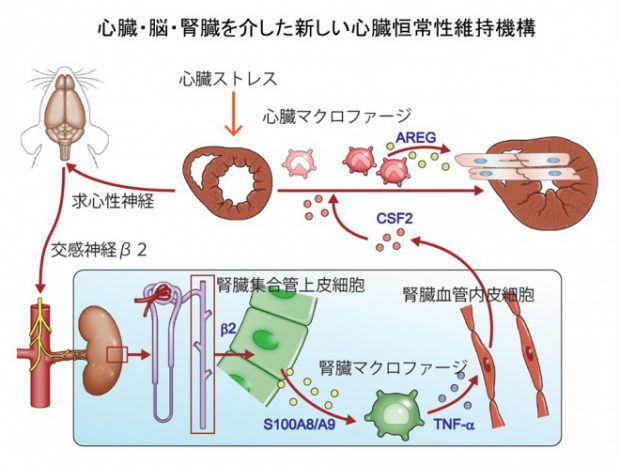
Fujiu K, Shibata M, Nakayama Y, Ogata F, Matsumoto S, Noshita K, et al. A heart-brain-kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat Med. 2017 May;23(5):611-22.
Ogata F, Fujiu K, Matsumoto S, Nakayama Y, Shibata M, Oike Y, et al. Excess Lymphangiogenesis Cooperatively Induced by Macrophages and CD4(+) T Cells Drives the Pathogenesis of Lymphedema. J Invest Dermatol. 2016 Mar;136(3):706-14.
Hachiya R, Shiihashi T, Shirakawa I, Iwasaki Y, Matsumura Y, Oishi Y, et al. The H3K9 methyltransferase Setdb1 regulates TLR4-mediated inflammatory responses in macrophages. Sci Rep. 2016 Jun 28;6:28845.
Tan X, Fujiu K, Manabe I, Nishida J, Yamagishi R, Terashima Y, et al. Choroidal Neovascularization Is Inhibited in Splenic-Denervated or Splenectomized Mice with a Concomitant Decrease in Intraocular Macrophage. PLoS One. 2016;11(8):e0160985.
Tan X, Fujiu K, Manabe I, Nishida J, Yamagishi R, Nagai R, et al. Choroidal neovascularization is inhibited via an intraocular decrease of inflammatory cells in mice lacking complement component C3. Sci Rep. 2015 Oct 28;5:15702.
Noda S, Asano Y, Nishimura S, Taniguchi T, Fujiu K, Manabe I, et al. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat Commun. 2014 Dec 12;5:5797.
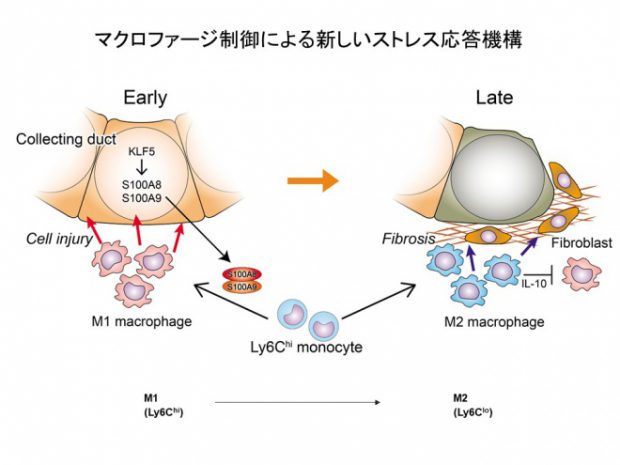
Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011 Sep;121(9):3425-41.
Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, et al. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010 Nov 16;122(20):2048-57.
Oishi Y, Manabe I, Tobe K, Ohsugi M, Kubota T, Fujiu K, et al. SUMOylation of Kruppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat Med. 2008 Jun;14(6):656-66.
Nishimura G, Manabe I, Tsushima K, Fujiu K, Oishi Y, Imai Y, et al. DeltaEF1 mediates TGF-beta signaling in vascular smooth muscle cell differentiation. Dev Cell. 2006 Jul;11(1):93-104.
Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005 Jan;1(1):27-39.
Fujiu K, Manabe I, Ishihara A, Oishi Y, Iwata H, Nishimura G, et al. Synthetic retinoid Am80 suppresses smooth muscle phenotypic modulation and in-stent neointima formation by inhibiting KLF5. Circ Res. 2005 Nov 25;97(11):1132-41.

 Nature Reviews Cardiologyでの解説
Nature Reviews Cardiologyでの解説
